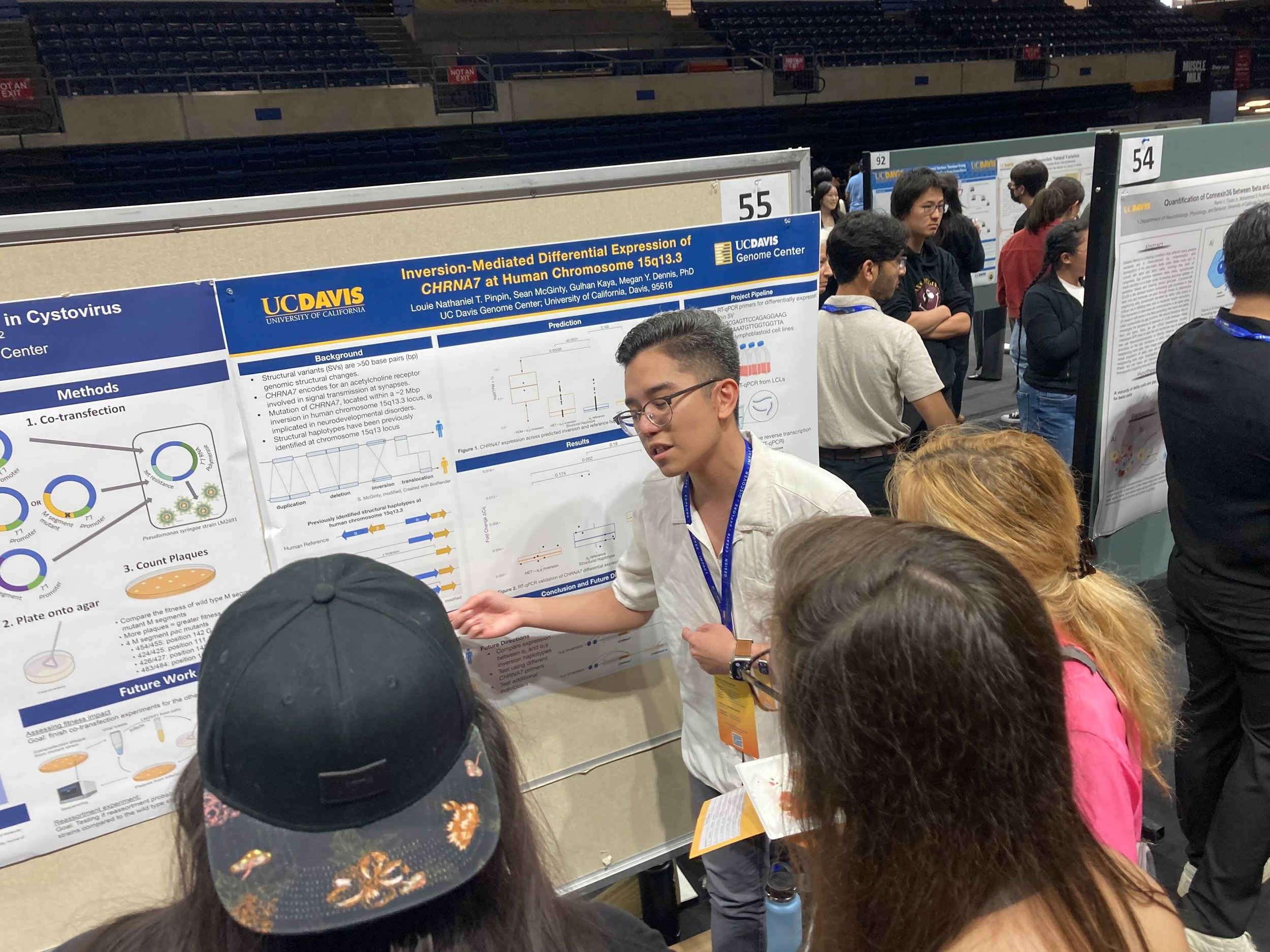
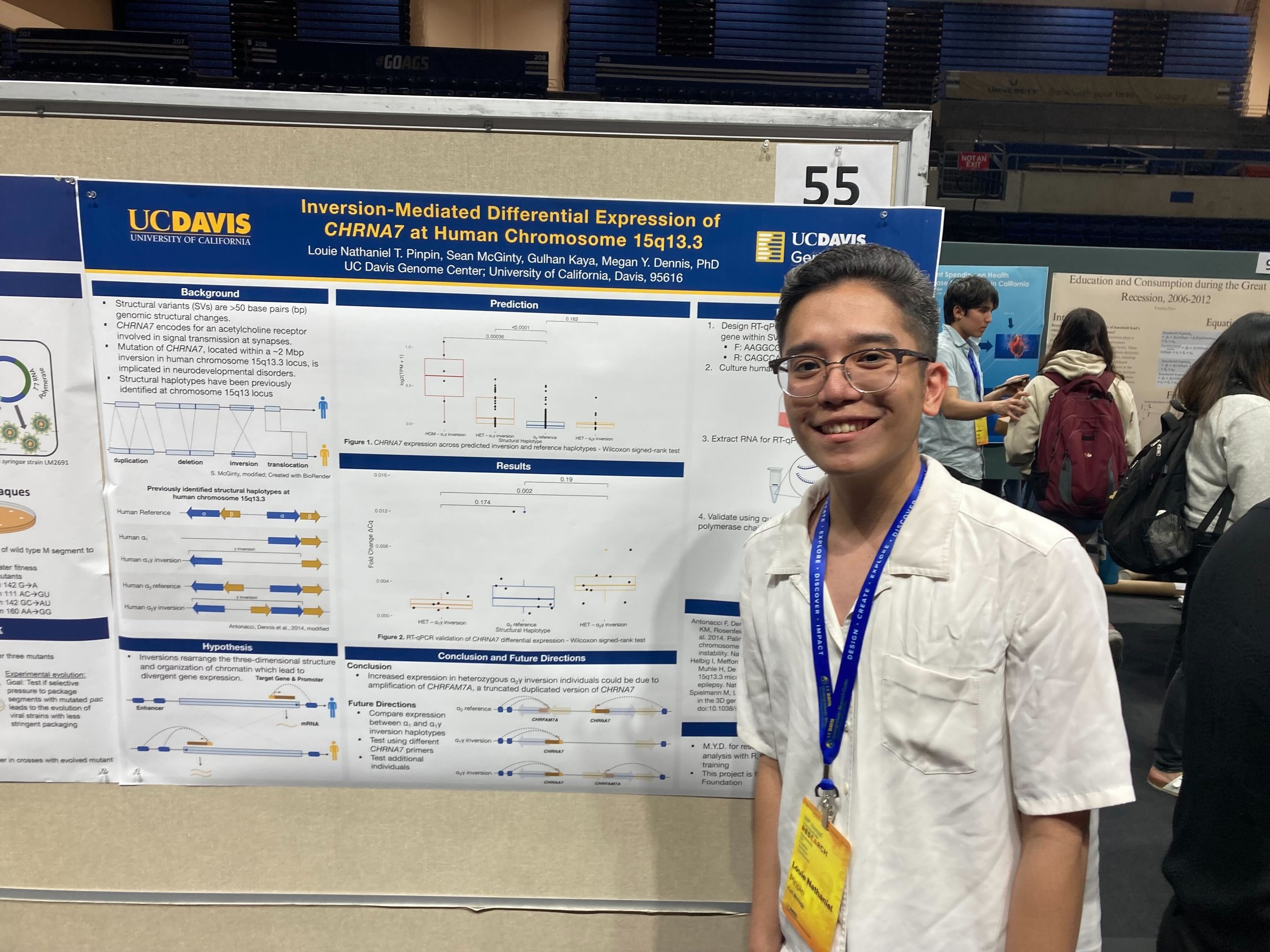
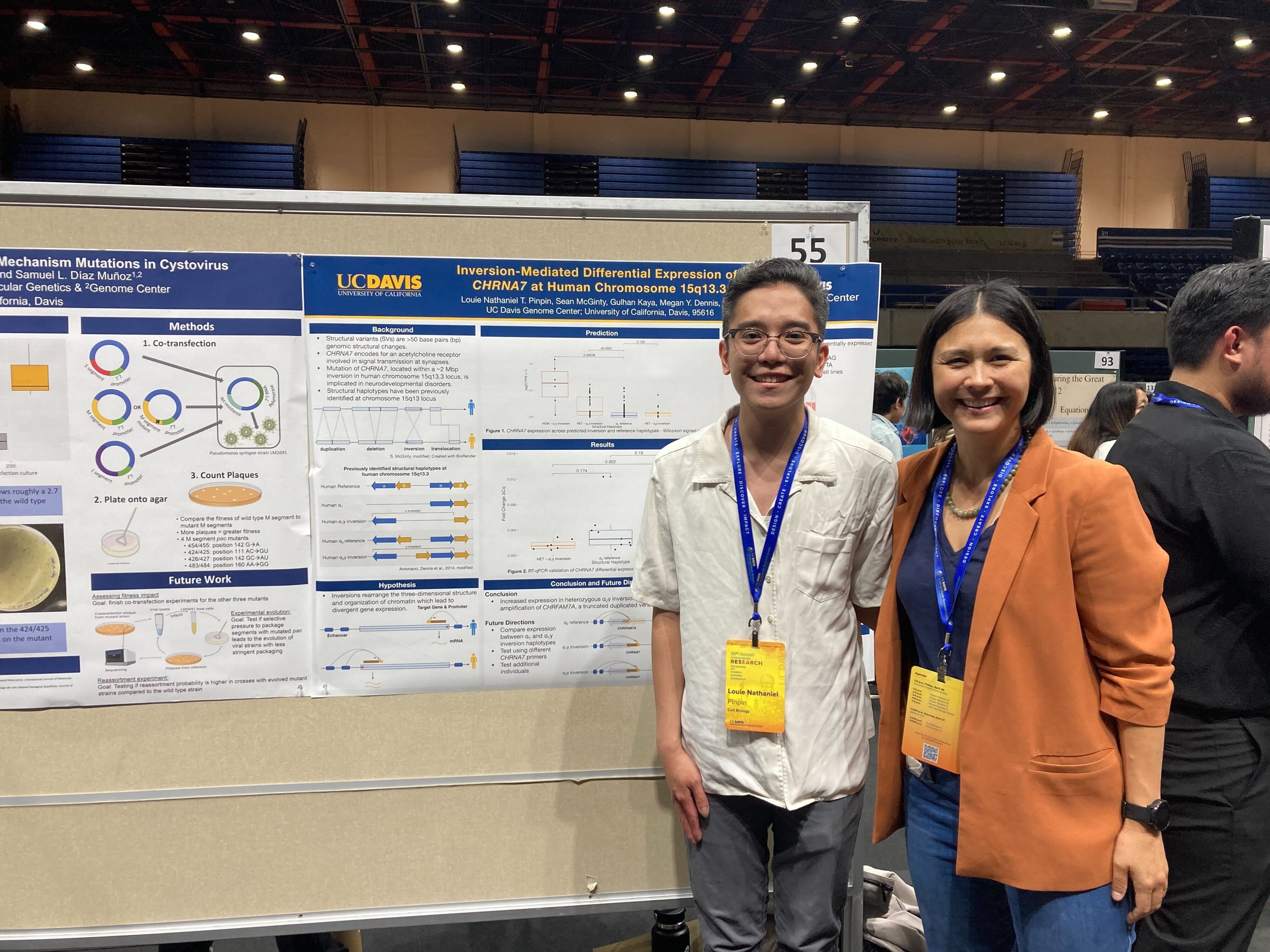
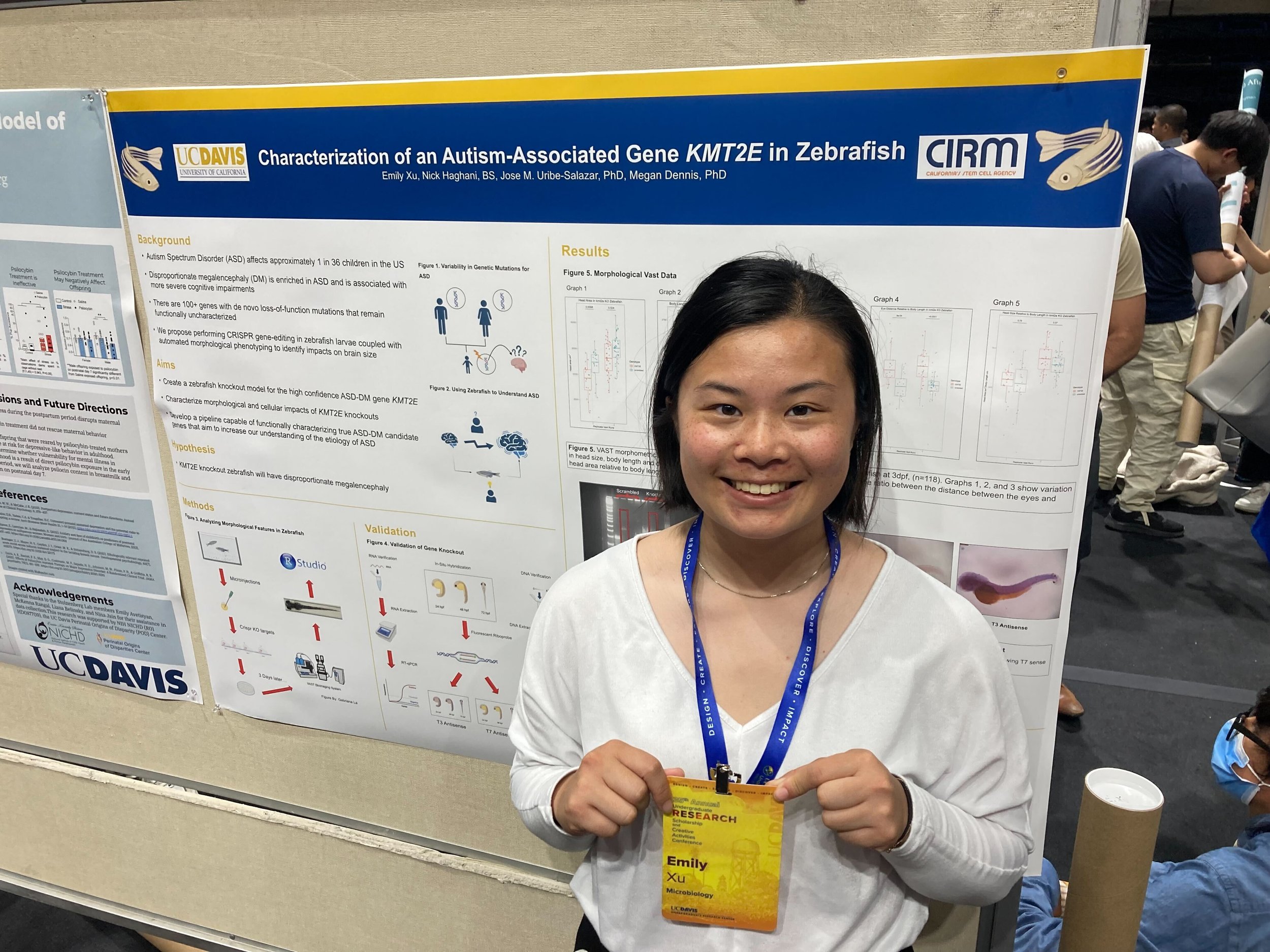
https://www.hhmi.org/news/hhmi-names-2024-hanna-gray-fellows
Congrats to former Dennis lab grad student and postdoc Daniela Soto for being named a 2024 HHMI Hanna Gray fellow. She is currently pursuing research at UC Santa Barbara co-mentored by Drs. Soojin Yi and Kirk Lohmueller (UCLA). We are so happy for you Dani!